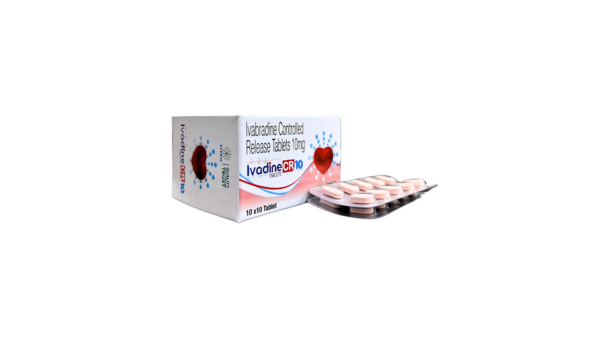
Ivabradine Controlled Release 10 mg Tablet – Heart Rate Management Simplified
Apr 15, 2025
Introduction
IVADINE CR 10 is a premium-quality pharmaceutical product containing Ivabradine Controlled Release 10 mg, designed for the treatment of chronic heart failure and stable angina. This formulation is intended to improve cardiac performance by selectively reducing heart rate without adversely affecting blood pressure or myocardial contractility.
Composition
-
Ivabradine Controlled Release 10 mg
Product Overview
Ivabradine 10 mg, offered as a controlled release (CR) formulation, is a unique heart rate-lowering agent that acts specifically on the sinoatrial node. Unlike beta-blockers or calcium channel blockers, it reduces heart rate without negatively impacting heart strength or vascular tone. This makes it especially beneficial for patients who are intolerant to other rate-control medications.
Ivabradine 10 mg Uses
IVADINE CR 10 is primarily used in the management of:
-
Chronic Heart Failure (with reduced ejection fraction)
-
Stable Angina Pectoris in patients with normal sinus rhythm who are inadequately controlled by or intolerant to beta-blockers
-
Heart Rate Reduction in patients requiring optimal cardiac output balance
Mechanism of Action of Ivabradine
The mechanism of action of Ivabradine is centered around its selective inhibition of the If current (funny current) in the sinoatrial node, the natural pacemaker of the heart. This inhibition slows the spontaneous pacemaker activity, effectively reducing the heart rate. Importantly, this does not influence myocardial contractility or blood pressure, allowing for symptom control without hemodynamic compromise.
Key Features of IVADINE CR 10
-
Controlled release for consistent plasma levels and prolonged action
-
Highly selective action on the sinus node
-
Does not impact myocardial contractility or systemic blood pressure
-
Reduces angina attacks and improves quality of life in heart failure patients
-
Helps prevent hospitalizations due to heart failure exacerbation
Dosage & Administration
-
The recommended starting dose is Ivabradine 10 mg once daily, or as directed by the physician
-
Should be taken with food to enhance absorption
-
Swallow the tablet whole; do not chew or crush
-
Monitor heart rate and rhythm periodically during treatment
Possible Side Effects
Most patients tolerate Ivabradine 10 mg well, but some may experience:
-
Bradycardia (slow heart rate)
-
Temporary visual disturbances (phosphenes)
-
Dizziness or tiredness
-
Irregular heartbeat (atrial fibrillation – rare)
Always consult your healthcare provider if any side effects occur.
Precautions & Warnings
-
Not recommended during pregnancy or breastfeeding without medical advice
-
Contraindicated in patients with severe liver dysfunction, acute heart failure, or significant bradycardia
-
Avoid grapefruit juice during therapy as it may increase drug levels
-
Use caution in combination with calcium channel blockers like verapamil or diltiazem
Frequently Asked Questions (FAQs)
Q1. What is tab Ivabradine 10 mg used for?
It is prescribed for chronic heart failure and stable angina to help reduce the heart rate and improve symptoms and outcomes.
Q2. How does Ivabradine work?
The Ivabradine mechanism of action involves inhibition of the If current in the sinoatrial node, leading to a slower heart rate without affecting blood pressure or heart muscle strength.
Q3. Can Ivabradine be used with other heart medications?
Yes, it is often used alongside beta-blockers, ACE inhibitors, and diuretics, but only under medical supervision.
Q4. What is the benefit of the controlled release formulation?
The CR (Controlled Release) form of Ivabradine 10 mg ensures steady plasma concentration, better tolerability, and sustained therapeutic effect throughout the day.
Q5. Is Ivabradine safe for long-term use?
Yes, when used under medical guidance, it is considered safe for long-term management of heart conditions.
Conclusion
IVADINE CR 10 (Ivabradine Controlled Release 10 mg) is a trusted choice for managing heart failure and angina pectoris. Its unique ability to selectively reduce heart rate without compromising cardiac output or blood pressure makes it an essential part of modern cardiology therapy. Whether as a standalone option or in combination with other agents, IVADINE CR 10 supports better cardiac function and enhances patient quality of life.
About The Author
Steris Healthcare Pvt Ltd, known as Sterispharma, was founded in February 2018 by a team of experienced professionals in the pharmaceutical industry. Headquartered in Navi Mumbai, the company holds certifications from WHO, GMP, and ISO, reflecting its dedication to maintaining high standards of quality and safety. Sterispharma’s mission is to provide affordable, high-quality medications across India, strictly adhering to WHO guidelines. With the convenience of an online pharmacy, customers can easily order medicines with home delivery options.
For further information
Email: info@sterispharma.com / contact@sterispharma.com
Call/WhatsApp: 8209542042 , 8824175417
News Referrences:
- Mumbai Based Steris Healthcare Announces Expansion Plan IPO Launch: msn.com
- Steris Healthcare Unveils Rs 50 Crore Expansion Plan, Eyes IPO In FY 2026-27: bwhealthcareworld.com
- Mumbai-based Steris Healthcare Pvt Ltd plans to launch IPO in FY 2026-27: indianstartupnews.com
- Steris Healthcare Announces Rs 50 Crore Expansion, IPO Planned For FY 2026-27: News18.com
- Steris Healthcare Pvt Ltd announces 50 crore expansion and IPO plans for FY 2026-- 27: expresspharma.com
- Steris Healtcare: Our Brand Is List On Trusted News Plateform.
- Steris Healthcare, a pharmaceutical company headquartered in Mumbai, has announced a strategic expansion plan worth 50 crore. The initiative includes setting up a new manufacturing facility in South India, doubling its current production capacity, and preparing for an Initial Public Offering (IPO) scheduled for the financial year 2026-- 27.
Having surpassed 100 crore in annual sales, Steris Healthcare aims to strengthen its nationwide footprint by expanding into key southern markets such as Kerala, Tamil Nadu, Telangana, and Karnataka. The upcoming manufacturing unit is expected to play a crucial role in enhancing the company's production capabilities and meeting the rising demand from both domestic and international markets.
Recent Post

Purifying Charcoal Face Wash for Oily and Acne-Prone Skin

Foaming Face Wash with Hydrating Power for Dry Skin Relief
TOP BETA GM: Clobetasol Propionate, Neomycin Sulphate & Miconazole Nitrate Cream.

Multi-Action Pain Relief Gel – Diclofenac, Linseed Oil, Menthol, Capsaicin & Methyl Salicylate

Best Sitagliptin 100 mg Brands In India | Steris Healthcare Pvt Ltd.

Nifedipine & Lidocaine Cream – Dual Relief for Anal Fissures & Hemorrhoids
Fexofenadine Hydrochloride 180 mg Tablet – Fast Relief from Allergies
ABIRASTERON 250 – Abiraterone Acetate IP 250 mg Tablet

PIMASERIN 34 – Pimavanserin 34 mg Tablet
CLAVAMOXY DS Oral Suspension – Amoxycillin 400mg & Clavulanic Acid 57mg